Thermal Decomposition of CaC2O4·H2O Studied by Thermo-Raman Spectroscopy with TGA/DTA | Analytical Chemistry
Whewellite, CaC2O4⋅H2O: structural study by a combined NMR, crystallography and modelling approach - CrystEngComm (RSC Publishing)

Experiment 8 - Precipitation of CaC2O4*H2O from the Salt Mixture Unknown name Moon Stardust Limiting - Studocu
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an
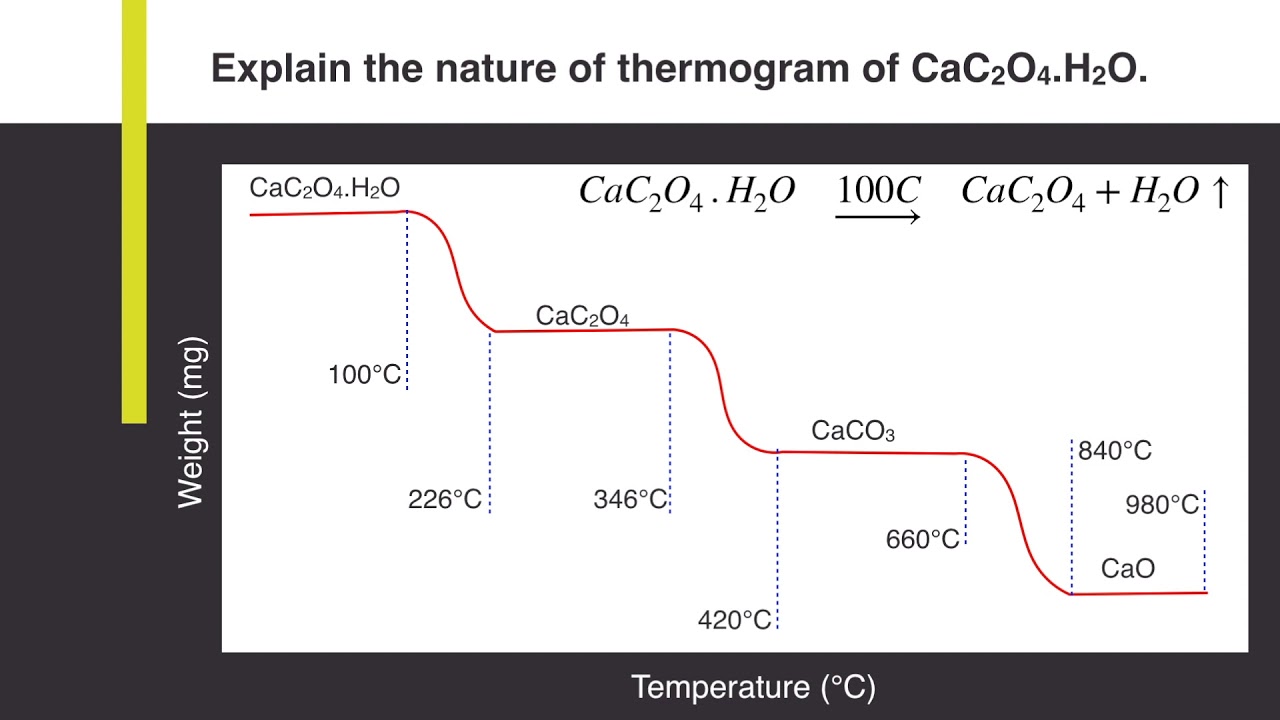
Explain the nature of thermogram of Calcium Oxalate Monohydrate (CaC2O4.H2O) | Analytical Chemistry - YouTube

A 0.60 g sample consisting of only CaC2O4 and MgC2O4 is heated at 500 ^∘ C , converting the two salts of CaCO3 and MgCO3 . The sample then, weighs 0.465 g .