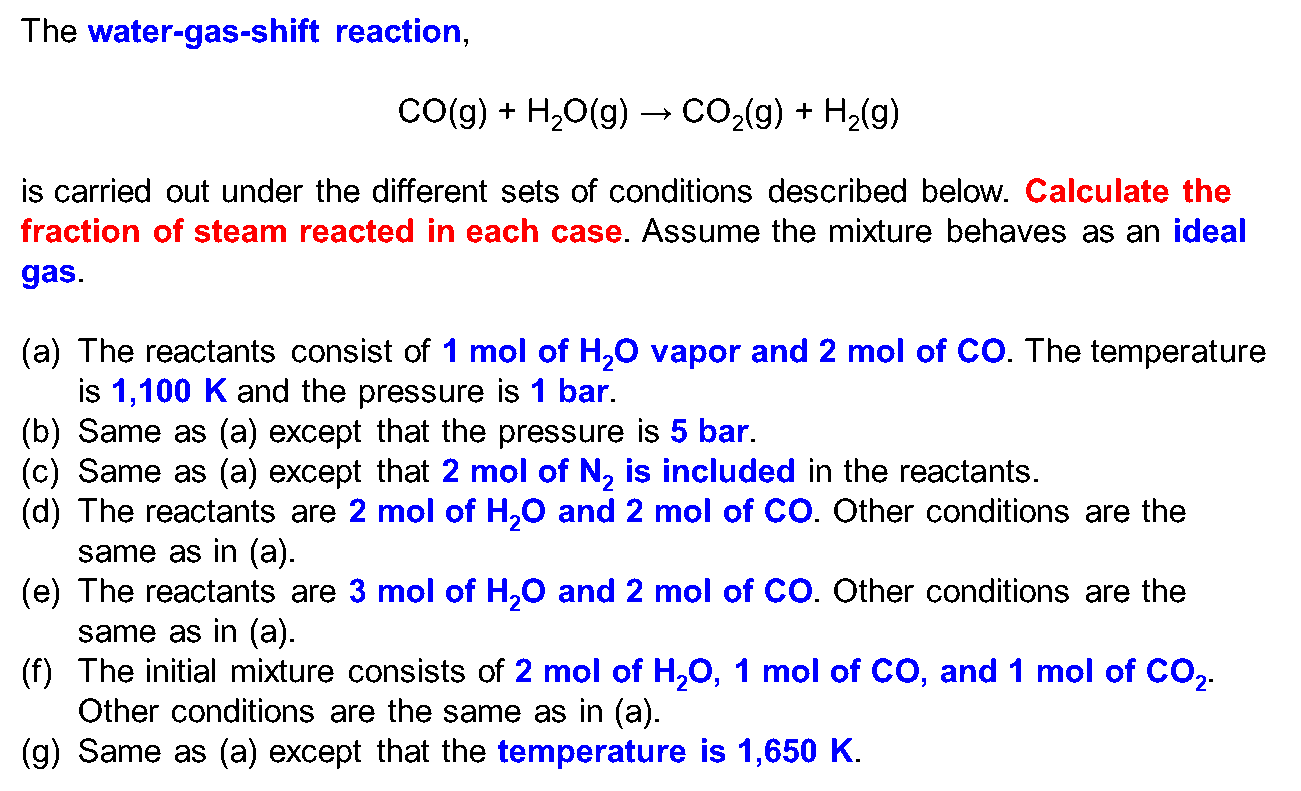
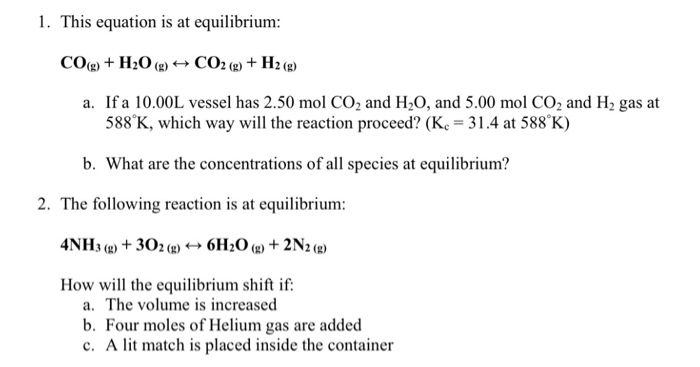
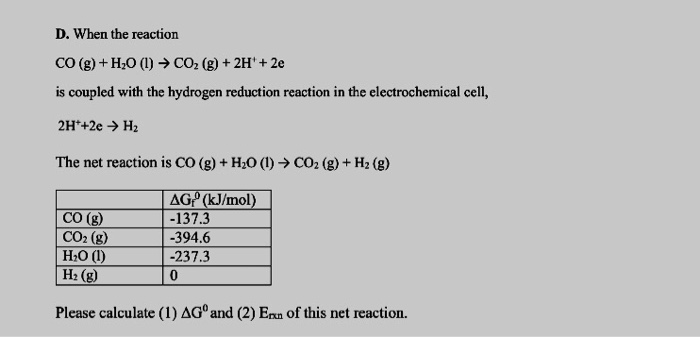
OneClass: 4) For the reaction H2(g) + CO2(g) H2O(g) + CO(g) at 700°C, Kp = 0.534. Calculate the numb...
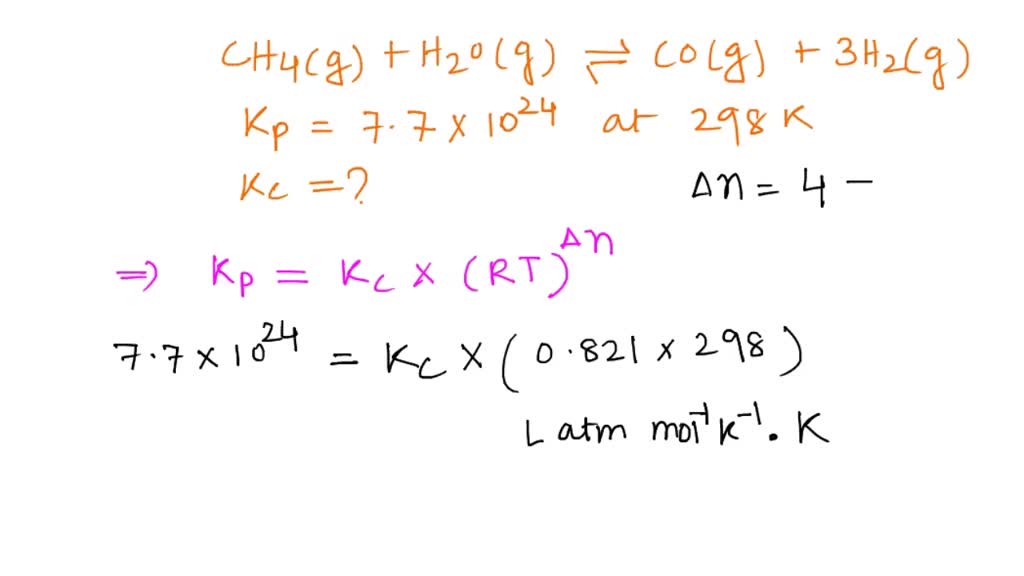
SOLVED: Consider the reaction below. H2O (g) + CH4 (g) <—> CO (g) + 3H2 (g) Kc = 4.7 at 1400 K What is Kp for this reaction at 1400 K? 6.2 x 104 4.7 8.2 x 10^8
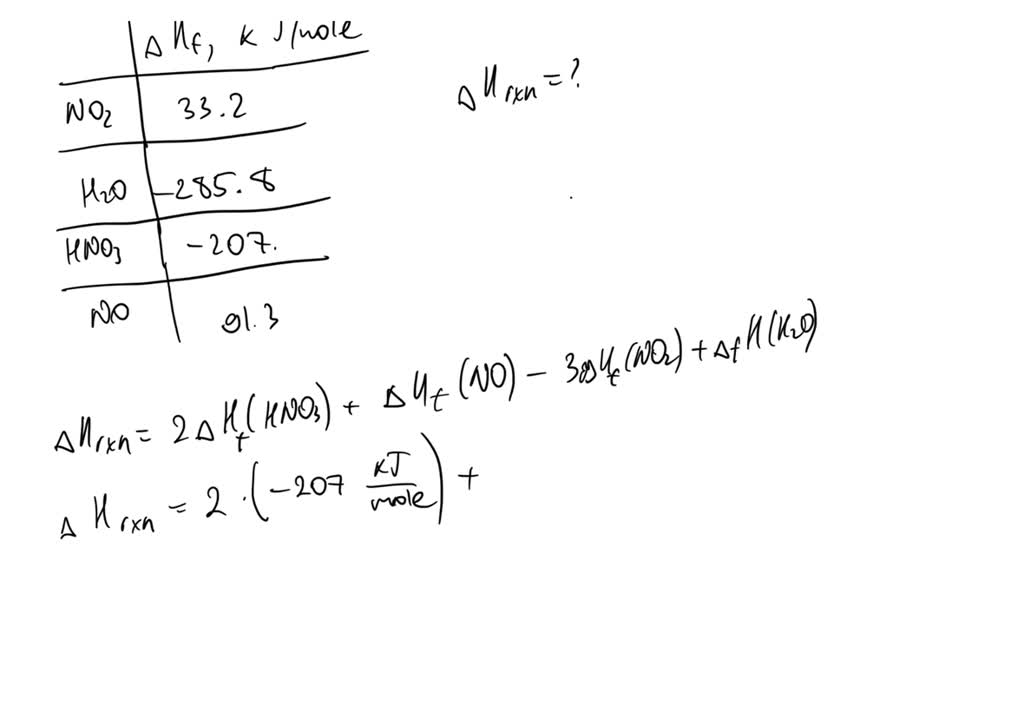
SOLVED: 4.) Consider the following reaction: 3NO2 (g) + H2O (l) ———–> 2HNO3 (aq) + NO (g) given the standard enthalpy of formations: NO2 (g) = 33.2 kJ/mole , H2O (l) = -
![SOLVED: Consider the reaction: CO(g)+H2O(g)⇌CO2(g)+H2(g) Kc=102 at 500 K A reaction mixture initially contains 0.130 M COand 0.130 M H2O. Part A What will be the equilibrium concentration of [CO]? [CO] = . SOLVED: Consider the reaction: CO(g)+H2O(g)⇌CO2(g)+H2(g) Kc=102 at 500 K A reaction mixture initially contains 0.130 M COand 0.130 M H2O. Part A What will be the equilibrium concentration of [CO]? [CO] = .](https://cdn.numerade.com/ask_previews/36628956-c06f-439b-80ff-a0f936ba3b67_large.jpg)