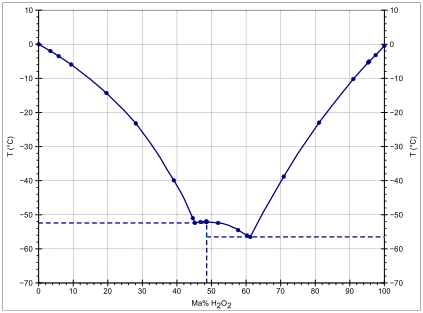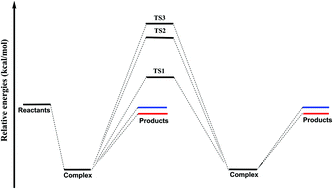
The water dimer reaction OH + (H2O)2 → (H2O)–OH + H2O - Physical Chemistry Chemical Physics (RSC Publishing)

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download

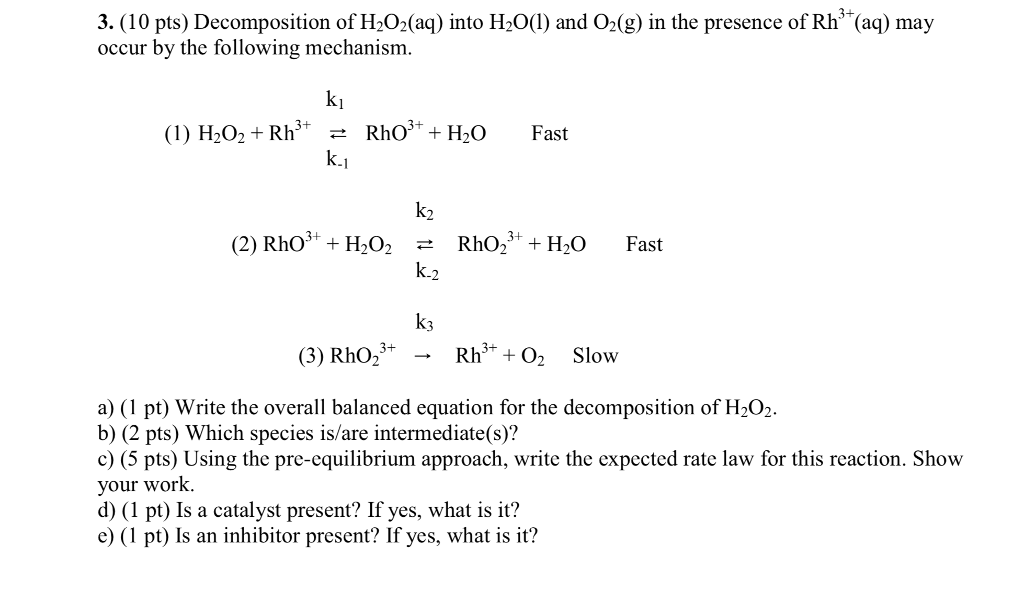
The decomposition of H2O2 has a strong thermodynamic driving force 2H2O2→ 2H2O + O2(g) Δ H = - 99kj/mole,Δ s = + 69JK^-1 mole^-1 Addition of solution of KI causes H2O2 to
, RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry RE(SO4)[B(OH)4](H2O), RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.7b02317/asset/images/large/ic-2017-02317c_0008.jpeg)
RE(SO4)[B(OH)4](H2O), RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry
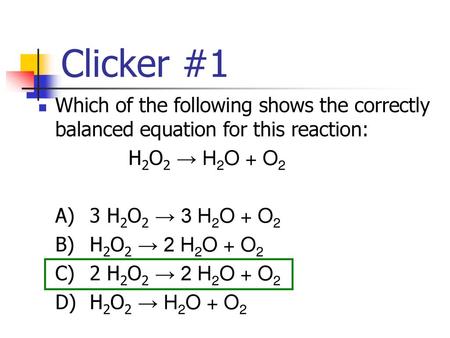
Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download
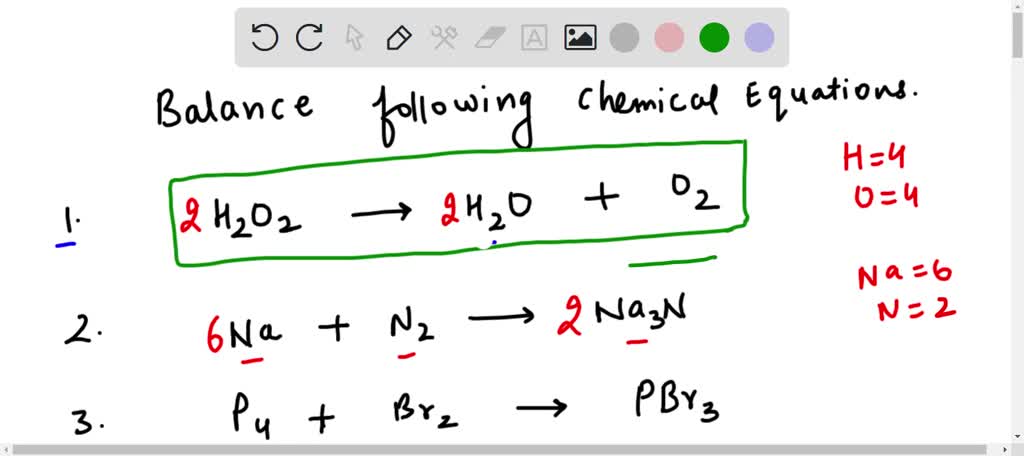

![For the reaction ; 2H2O2(aq)→ 2H2O(l) + O2(g) , rate of decomposition for H2O2 = k[H2O2]^2 For the reaction ; 2H2O2(aq)→ 2H2O(l) + O2(g) , rate of decomposition for H2O2 = k[H2O2]^2](https://dwes9vv9u0550.cloudfront.net/images/2785739/3a8762db-6023-4a5c-9588-862513a5c19d.jpg)




![Water [H2O] Molecular Weight Calculation - Laboratory Notes Water [H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/water-molecular-weight-calculation.jpg)