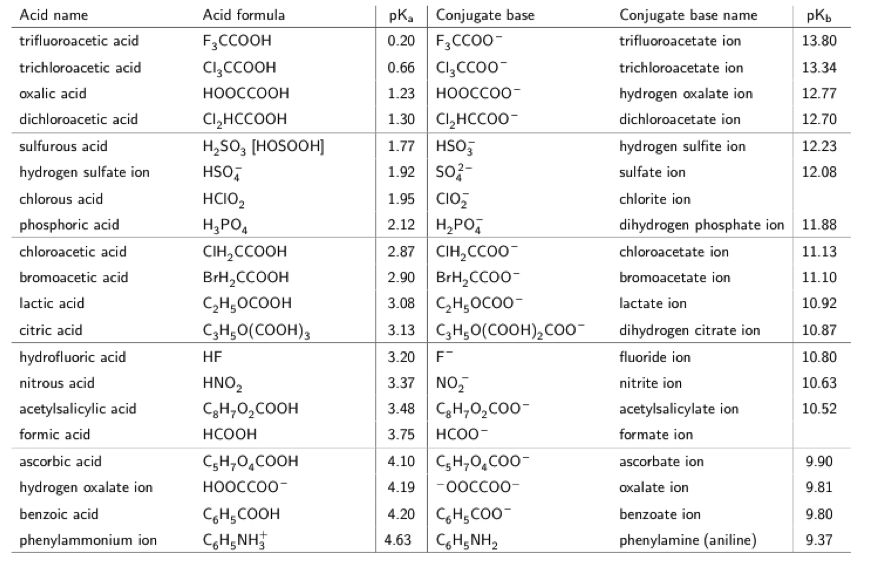
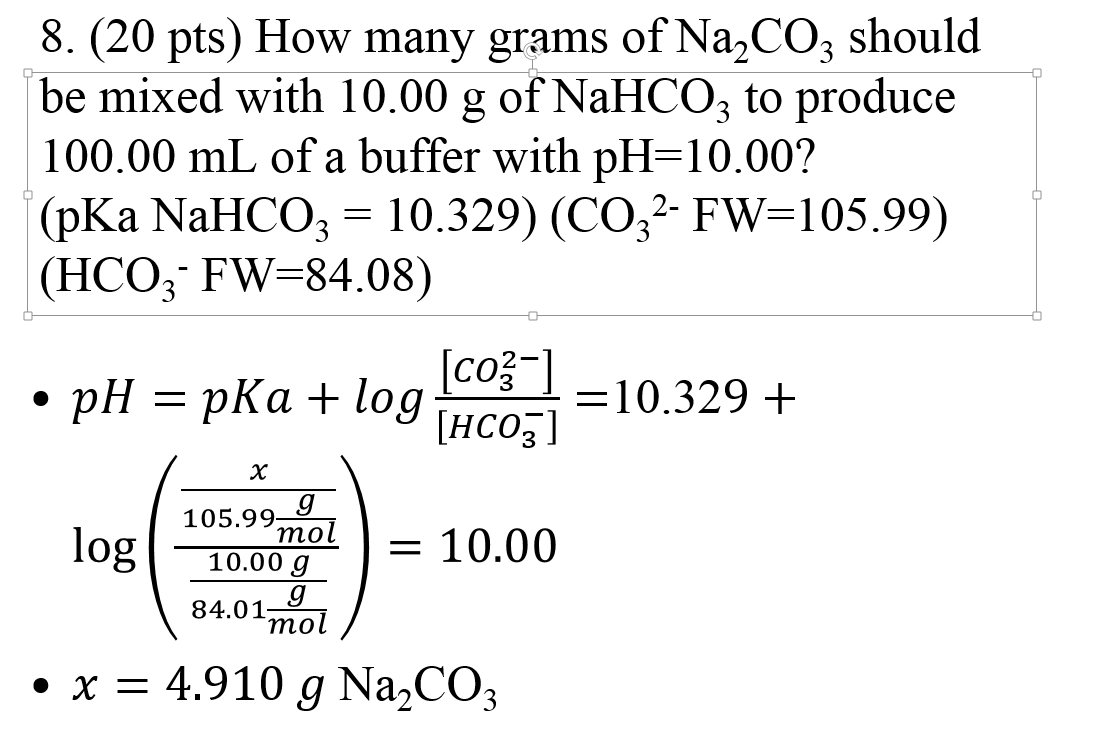![50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ] 50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/OHZYS2ZrRHZKams=/sd/)
50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ]
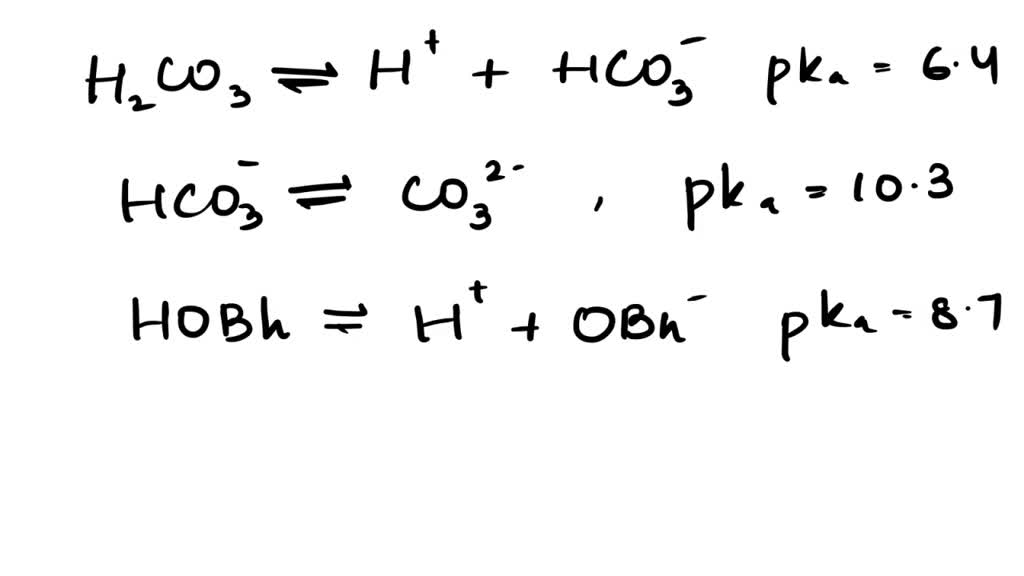
SOLVED: The pKas of H2CO3 are 6.4 10.3. The pKa of HOBr is 8.7. If equimolar amounts of Na2CO3 and HOBr are dissolved in water what will be the predominant anionic species
Synthesis of Unsymmetrical Diaryl Acetamides, Benzofurans, Benzophenones, and Xanthenes by Transition-Metal-Free Oxidative Cross

physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange

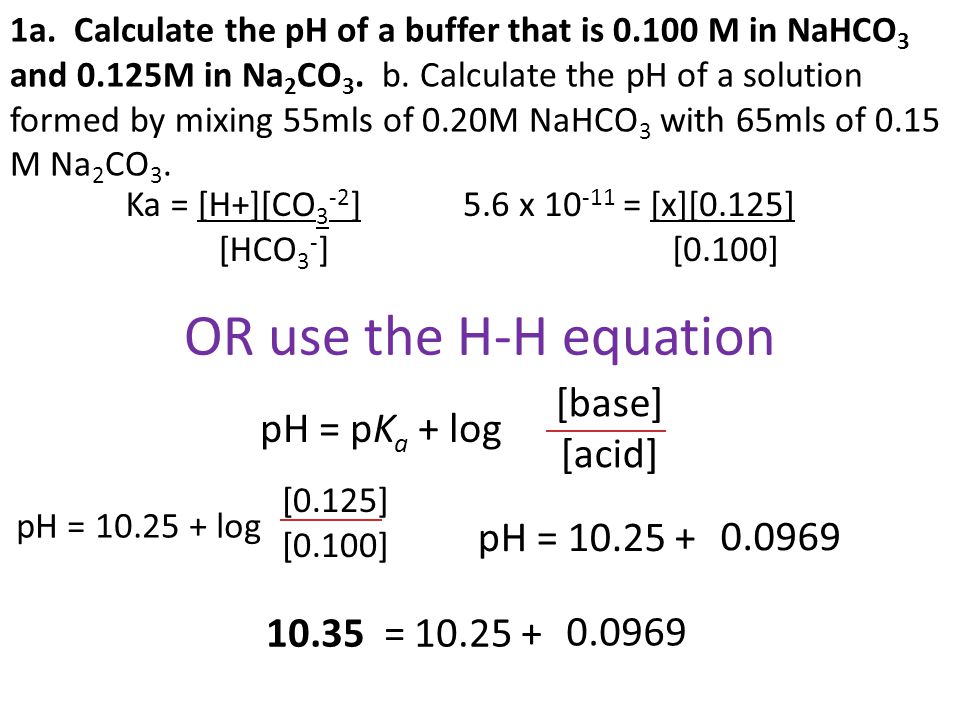
The pKa of a weak acid, HA, is 4.80. The pKb of a weak base, BOH, is 4.78. What is the pH of an aqueous solution of the corresponding slat BA?
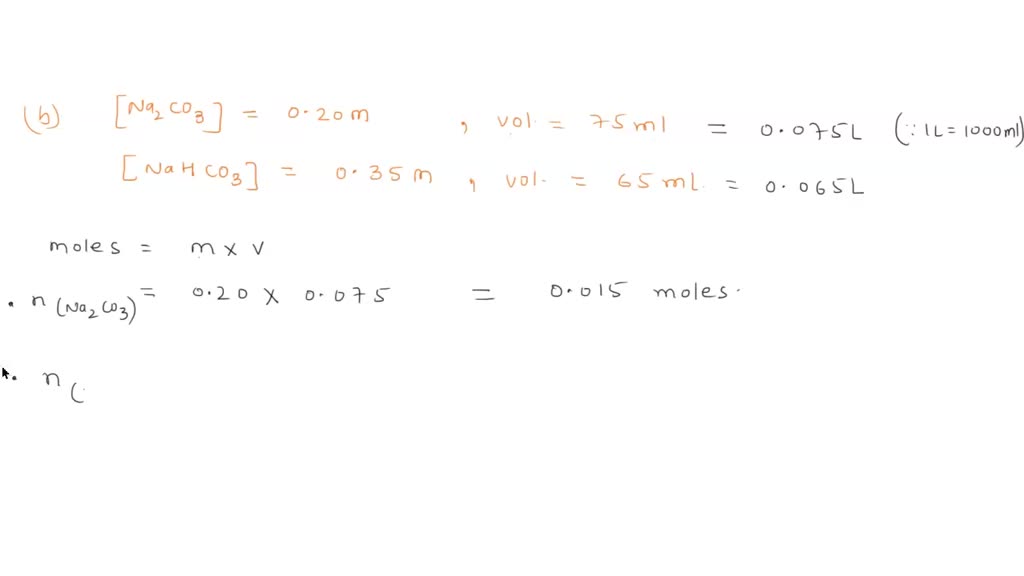
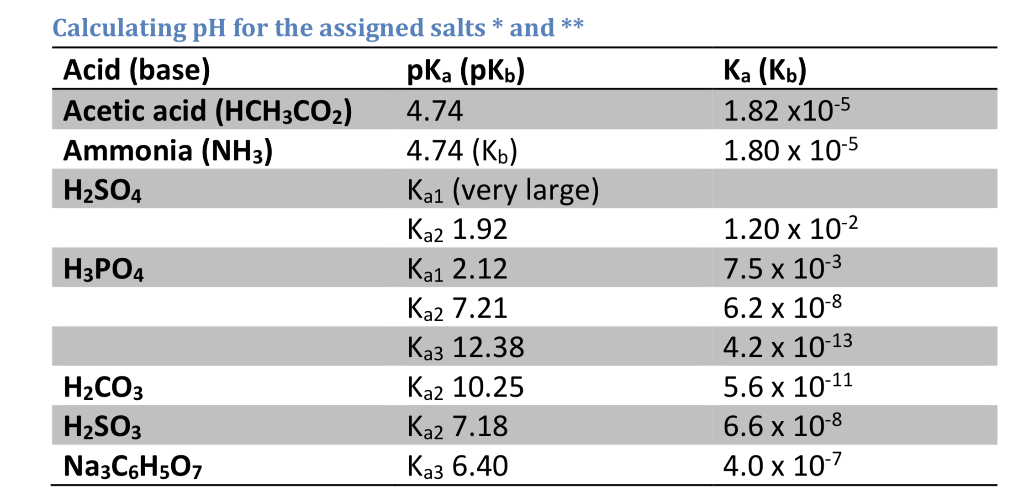
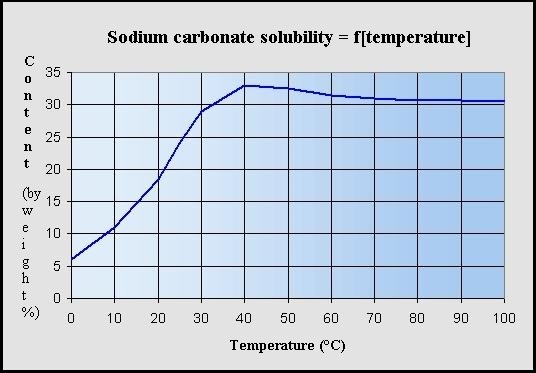
SOLVED: Consider a buffer solution that contains 0.35 M NaHCO3 and 0.25 M Na2CO3. pKa(HCO3-)=10.33. If the acceptable buffer range of the solution is ±0.10 pH units, calculate how many moles of
![50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ] 50 mL of 0.05 M Na2CO3 is titrated against 0.1 M HCl . On adding 40 mL of HCl , pH of the solution will be[Given for H2CO3, pKa1 = 6.35; pKa2 = 10.33; log 3 = 0.477 , log 2 = 0.30 ]](https://dwes9vv9u0550.cloudfront.net/images/1766662/06af4a5d-9ecd-450c-9b4d-b90fc83566c2.jpg)

![The pKa values for various precipitants [17]. | Download Scientific Diagram The pKa values for various precipitants [17]. | Download Scientific Diagram](https://www.researchgate.net/publication/339359335/figure/tbl1/AS:860297669640196@1582122356901/The-pKa-values-for-various-precipitants-17.png)